An Opportunity in a $36 Billion Dollar Industry That Nobody is Talking About
By: BioCorRx Inc.

The addiction epidemic makes headlines left and right. A staggering 23.5 million Americans are addicted to drugs and alcohol.
The cost of substance abuse to our nation exceeds $740 billion annually and is hurting communities, families, and the people we care about.
There are business opportunities related to the addiction epidemic that offer vast potential and we believe one of these opportunities may be the addiction treatment industry. The growth potential in this arena could be staggering as it is estimated that 1 out of every 10 Americans has a substance abuse problem.
The $36 billion addiction treatment industry is undergoing a radical transformation and we believe that we, BioCorRx (OTCQB:BICX), are staying well ahead of this transformation.

BioCorRx has TWO distinct business models that we believe will not only position us at the forefront of fighting substance addiction, but also shine a light on an industry shift towards combining medication and counseling:

The Future of Addiction Treatment
The way addiction is treated today has evolved greatly compared to 100 years ago. Treatments were once comparable to torture, and many people with addictions were hastily thrown into prisons or asylums, assuming they lacked strong moral fiber and were mentally weak.
Addiction is a growing concern across the globe with a recorded 230 million drug addicts worldwide according to the Huffington Post. America accounts for a tenth of them!
The business of recovery has opened the door for BioCorRx and its unique recovery program, which could potentially help many Americans fight their addiction.

Designed to treat alcoholism and certain opioid addictions, the BioCorRx® Recovery Program is used by a network of independently owned and operated treatment centers located throughout the United States.
BioCorRx's program utilizes our worldwide rights (except in Australia and New Zealand) to a proprietary implant formulation of the FDA-approved drug, naltrexone. We believe this implant formulation is a highly effective utilization of naltrexone which has proven to substantially reduce cravings for drugs and alcohol.
We believe our recovery program is high-quality, comprehensive, and cost- effective, It was built on a strong foundation of experience and practice, allowing for an improved quality of life for recovering addicts.
Specifically formulated, completely biodegradable pellets are typically inserted just beneath the skin in the lower abdominal area for a slow and steady release of medication. The implant allows for naltrexone to release into the bloodstream in a sustained dosing pattern over time and lasts, depending on individual factors, for several months.
This allows the patient the opportunity to reset their behavioral patterns by engaging in therapy without the constant bombardment of cravings for opioids or alcohol, thus, we believe, dramatically increasing the overall likelihood of success.
BioCorRx is currently seeking FDA approval of new formulations of naltrexone with anticipated approval of one or more formulations by early 2020.
Additionally, commercialization for a naltrexone implant is focused on a more rapid and cost effective 505(b)(2) regulatory pathway.
Our BioCorRx® Recovery Program, which combines medication and therapy, is already producing revenue from independent treatment centers using the program. The Company is also working on private/public partnerships with municipalities including Philadelphia and Anaheim.
The program has also been used in lieu of conviction in Ohio for an individual as previously mentioned in a company press release, and the Company has just begun a new partnership with CereCare as an authorized distributor of the program as well.
With what we believe is our solid balance sheet and clean capital structure, BioCorRx is positioned to grow exponentially as our product advances in the market and our new formulation advance through the regulatory pathway.

The Facts
- 38 million Americans are heavy drinkers, according to the CDC
- Per the CDC, more than 12% of the population are alcohol dependent
- 5 million American adults according to the Substance Abuse and Mental Health Services Administration's (SAMHSA's) National Survey on Drug Use and Health are addicted to drugs and or alcohol
- 100 Americans die from preventable overdose every day, per the CDC
- 770% increase in those seeking treatment for opioid addiction in the last 15 years
- In 2010, more than 12 million people reported using pain killers non-medically
- 85% of the people with alcohol abuse or dependence go untreated
- Heroin is the most widely used opiate according to the National Survey on Drug Use and Health with more than 900,000 estimated users
With nearly 900,000 people dying from alcohol‐related causes every year in U.S., there is a huge push to increase treatments.
Drug abuse is also steadily on the rise. Over 2 million people in the U.S. abuse prescription pain relievers. Heroin is the most widely known illegal opiate, with users estimated as high as 900,000 Americans.
According to the CDC, drug overdose was the leading cause of accidental death in 2015.

Addiction Treatment Spending
Addiction treatment spending has grown faster than:
- the total GDP inflation rate
- the medical care inflation rate
- population growth.
From 2008‐ 2014, growth in addiction treatment spending also exceeded growth in total health spending.

In 2015, addiction treatment spending was $36 billion.
- Largest payors were state and local funds‐ 29% or almost $10 billion
- Medicaid was second largest payor‐ 21% ($7.2 billion)
- Private Insurance‐ 18% ($6.1 billion)
Furthermore, the care setting costs are equally astronomical:
- Outpatient care $14.4 billion
- Residential care $9.72 billion
- Inpatient care $6.64 billion
- Prescription drugs $1.8 billion in 2015
Right now, there is a strong shift towards combining medication and counseling, and we believe the BioCorRx® Recovery Program is on the forefront of Medication-assisted Treatment (MAT).
Former U.S. Surgeon General Dr. Vivek Murthy:
"By its very definition, medication‐assisted treatment has to include more than medication alone. It has to include counseling services and the other support services that are an important part of effective addiction treatment; that's an important point because many people do not recognize that aspect of MAT. It does not mean that you can take a pill for a couple of weeks and be cured of your substance use disorder. That's not actually how it works; in the same way that if you have diabetes, you don't take a pill for a couple of weeks and you're cured of your diabetes. It's a chronic illness that requires long‐term management, and the same is true of addiction."
The BioCorRx® Product Pipeline
Naltrexone Implant BICX102: for the treatment of opioid and alcohol use disorders
- Acquired North American rights to new implant formulations and Prodetoxone® study data in 2016.
- Extended release implant providing a therapeutic effect and maintaining plasma levels approx. 90 days.
- 3-month formula has been used in Russia for several years with longer version just developed.
- Pre‐IND meeting held with FDA January 24, 2018
- Data acquired for Prodetoxone, which is one of only two known naltrexone implants approved by a regulatory body (Prodetoxone has been approved in Russia for 15+ years and another naltrexone implant was approved in Georgia)
- Prodetoxone been through multiple trials conducted at St. Petersburg Scientific‐Research Center of Addictions and Psychopharmacology, Pavlov Medical University, in conjunction with the University of Pennsylvania, Department of Psychiatry, Philadelphia, USA
- Formulas and protocols that we believe are very similar to Prodetoxone were purchased along with the North American rights to certain non‐public Prodetoxone study data which is expected to assist in a more efficient FDA approval pathway.
Naltrexone Injectable BICX101:for the treatment of opioid and alcohol use disorders
- Partnered with TheraKine to complete development of a new injectable version of naltrexone using underlying patented and patent pending technology
- Utilizing TheraKine's patented micro‐delivery technology
- Goal to be delivered subcutaneously (SQ) or intramuscularly (IM) in smaller muscle (deltoid)
- Anticipate cost efficient, outsourced manufacturing
- Ability to sub‐license
- Product in formulation development phase
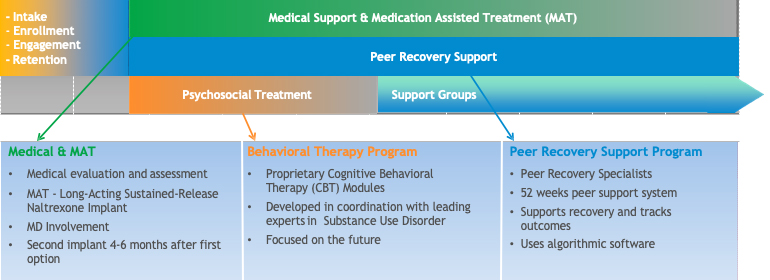
The BioCorRx® Recovery Program
Proprietary Naltrexone Implant
- Used per patient prescription under state and federal compounding rules
- Implant inserted in fatty tissue of abdomen
- Simple outpatient procedure by licensed medical professional
- Procedure only takes 20‐30 minutes and begins to work within hours
- Substantially reduces cravings for drugs and alcohol for several months
Proprietary Cognitive Behavioral Therapy (CBT) Program/Peer Support/Tracking
- Patients complete 35 treatment modules during 16 private sessions, typically in under 90 days
- Step‐by‐step approach for specific addiction and can include family and friend participation
- Therapists readily available
- 12-month peer recovery support in conjunction with, or after counseling
- Modules now available to patients via mobile app
BioCorRx® Recovery Program is distributed by partner clinics across the US
- Fees are paid to BioCorRx per program sold by independent treatment providers
- Approximately 18 partner clinics currently and growing
- Discussions being held to incorporate all or portions of the program into traditional residential treatment centers
BioCorRx has already been featured in USA Today, The Doctor Oz Show, MSNBC, Fox News, Fox Business Channel, and many other local news segments across the country.
We believe we have an innovative approach, combining medication with supportive behavioral management to treat substance abuse.
This is a vast market that could represent an unparalleled growth opportunity for BioCorRx as it offers a solution to many people who have addiction problems.
Safe Harbor Statement
The information in this article includes forward-looking statements. These forward-looking statements generally are identified by the words "believe," "project," "estimate," "become," "plan," "will," and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties. Although the Company believes that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof.
